Abstract
Myelofibrosis (MF) is a life-threatening myeloproliferative neoplasm. The Janus Kinase inhibitors (JAKi) ruxolitinib, pacritinib and fedratinib are FDA-approved treatment options for MF. Available JAKi treatments block the JAK-STAT signaling pathway, dysregulated in patients with MF, resulting in relief of splenomegaly and debilitating MF-related constitutional symptoms in patients. However, inhibition of this signaling pathway has limited activity on disease-altering effects because it does not eliminate malignant clonal stem cells that drive disease progression (Greenfield, 2018).
Imetelstat is a first-in-class telomerase inhibitor that has shown meaningful clinical improvement in IMbark, a Phase 2 study in patients with intermediate 2 (Int2) or high-risk MF whose disease has relapsed after or is refractory to JAKi (Mascarenhas JCO 2021; NCT02426086). Treatment with 9.4 mg/kg imetelstat resulted in 32.2% symptom response (total symptom score reduction ≥50%) at Week 24 and median overall survival (OS) of 29.9 months with median overall study follow-up of 27.4 months. Dose-dependent inhibition of telomerase with imetelstat resulted in on-target activity that correlated with clinical benefits. Also, a dose-dependent reduction in variant allele frequency of MF driver mutations indicated targeting of the underlying malignant clone. While this Phase 2 study supports the efficacy of imetelstat in patients no longer benefiting from JAKi treatment, preclinical studies have demonstrated that combination treatment of MF patient-derived xenograft mice with ruxolitinib followed by imetelstat results in greater disease reduction than in mice treated with either agent alone, while sparing normal hematopoiesis (Hu 2019). Thus, combining the non-overlapping mechanisms of action of ruxolitinib (a JAKi) and imetelstat (a telomerase inhibitor) may not only improve disease symptoms but also may alter the natural history of MF through activity on malignant clonal stem cells.
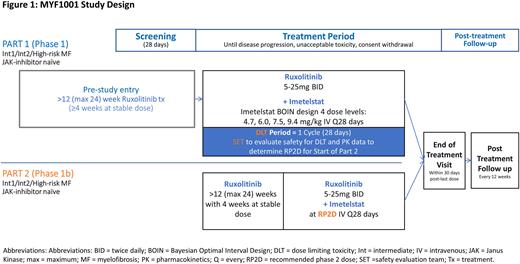
Study MYF1001 (IMproveMF; NCT05371964) is an open label, single arm study combining ruxolitinib and imetelstat as a frontline therapy in patients with Dynamic International Prognostic Scoring system Int1, Int2 and high-risk MF. Eligible patients will have an Eastern Cooperative Oncology Group Performance Status score of 0-2 and peripheral blood and marrow blast counts <10%. Patients cannot have clinically significant cardiovascular disease, active systemic hepatitis infection, chronic liver disease unrelated to underlying MF or a prior history of a hematopoietic stem cell transplant or splenectomy. To allow each patient to reach an individualized stable dose of ruxolitinib and mitigate the risk of thrombocytopenia observed upon initiation of both ruxolitinib and imetelstat, single agent ruxolitinib treatment is required for at least 12 weeks (maximum 24 weeks), with a stable dose for 4 consecutive weeks immediately preceding addition of imetelstat. In Part 1 (Phase 1, dose escalation portion), 4 escalating intravenous doses of imetelstat (4.7, 6.0, 7.5, and 9.4 mg/kg) will be investigated every 28 days in ~21 patients to determine the recommended phase 2 dose (RP2D) based on a Bayesian optimal interval design (Figure 1). Three patients will be enrolled at each imetelstat dose level in a step wise manner, with progression to subsequent dose levels determined by the presence of dose limiting toxicities and review and approval from the Safety Evaluation Team. In Part 2 (Phase 1b, dose combination and expansion portion), ~20 patients will undergo open label, single arm combination treatment of ruxolitinib followed by imetelstat at the RP2D established in Phase 1.
The primary objectives are to determine the RP2D of imetelstat, when administered in combination with individually optimized doses of ruxolitinib for Part 1, and to evaluate safety and preliminary clinical activity of this combined treatment regimen for Part 2. Secondary objectives include symptom and spleen response rates at Week 24, progression-free survival, clinical response assessments per modified 2013 International Working Group - Myeloproliferative Neoplasms Research and Treatment criteria, time to and duration of response, reduction in degree of bone marrow fibrosis, and the pharmacokinetics and immunogenicity of imetelstat.
The Phase 1 dose-escalation (Part 1) is open for enrollment, with approximately 3 sites planned in North America.
Disclosures
Bradley:Geron Corporation: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Komrokji:Acceleron Pharma: Consultancy; Geron: Consultancy; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Speakers Bureau; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Berry:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Dougherty:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Sherman:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Peng:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Huang:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Wan:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Feller:Geron Corporation: Current Employment, Current equity holder in publicly-traded company. Mascarenhas:Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; Janseen: Research Funding; Merus: Research Funding; Forbius: Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Galecto: Consultancy; Merck: Research Funding; AbbVie: Consultancy, Research Funding; GSK: Consultancy; Sierra Oncology: Consultancy; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal